Background and Motivation
IBS and POI
Irritable bowel syndrome, commonly known as IBS, is a functional gastrointestinal (GI) disorder that is characterized by chronic abdominal discomfort and irregular bowel habits. An estimated 15% of the adult U.S. population has IBS but only 5-7% receive a diagnosis (American College of Gastroenterology, 2023). IBS patients have a higher prevalence of depression which may be attributed to their lower quality of life as they frequently suffer chronic pain (Kopczyńska, 2018). Currently, IBS is diagnosed based on symptoms and medical history rather than having a landmark-based diagnosis. However, there is lack of knowledge of IBS symptomology and how abnormal colonic motility patterns are related to symptoms. This lack of knowledge leads to frequent guesswork when it comes to diagnoses and treatment. There are different methods for diagnosing IBS, including using blood tests, x-rays, and occasionally colonoscopies to check for inflammatory bowel disease (IBD) (U.S. Department of Health and Human Services, 2017). Current research on colon motility patterns in IBS requires invasive intracolonic high-resolution manometry (HRM), where catheters are placed throughout the colonic wall via a colonoscopy (Li et al, 2019) (Wiklendt et al, 2023). In addition to the invasiveness of this research method, it also comes at a high cost which restricts its repeatability.
Further, individuals with IBD and colon cancer frequently undergo colon surgery as a form of treatment (Johns Hopkins University, n.d.). Unfortunately, 5-30% of patients experience post-operative complications known as post-operative ileus, so patients are often asked to remain in the hospital for monitoring (Buchanan & Tuma, 2023). Studies show that normal colonic function should show within 72 hours post-operation, meaning patients will be further burdened with high hospital expenses and time off of work.
Experiment protocol
Dr. Jon Erickson’s gastrointestinal systems research measures and maps colonic motility patterns. Colonic signal data from subjects with IBS is collected to eventually find an alternative noninvasive method to research IBS.

We investigate colonic signal to monitor meal responses to expand upon current HRM invasive studies that characterize normal motility patterns by inducing eating in subjects with and without IBS (Lin et al 2017). In our lab, we ask subjects to fast overnight and only drink water after dinner the night before the study. During the study, colonic signal data is recorded by connecting electrodes to subjects’ lower left abdomen (Figure 1) to monitor activity in the sigmoid region of the colon, as prior research has determined higher sigmoidal activity in subjects with IBS (Lin et al., 2017). Subjects are asked to sit in a reclined position and limit movement for the duration of the study to limit motion artifacts in the recorded signal. First, a baseline recording is collected for around 1-1.5 hours, then the subject is provided a meal that is considered to be standard according to their typical diet. Then, data recording continues for another 1-1.5 hours. If data collection could be extended to 24 hours or longer, more information could be extracted to better understand the traits of IBS, like occurrences to be monitored such as waking or napping (Bassotti et. al, 1993).
Meal Responses: Heathy vs Non
After gathering the colonic signal data, it is subsequently filtered and compared against a “healthy normal” (those without IBS) data set. An example of filtered electrical signal, and the resulting spectrogram can be seen in Figure 2, which shows the meal start and the postprandial signals. Cyclic motor patterns (CMPs) are stimulated by meal intake and tend to be concentrated in the rectosigmoidal region (Figure 3), where they can then be detected via body surface electrical recordings (Dinning, 2014). In those with IBS, shorter meal responses tend to be seen, like that shown in Figure 4.
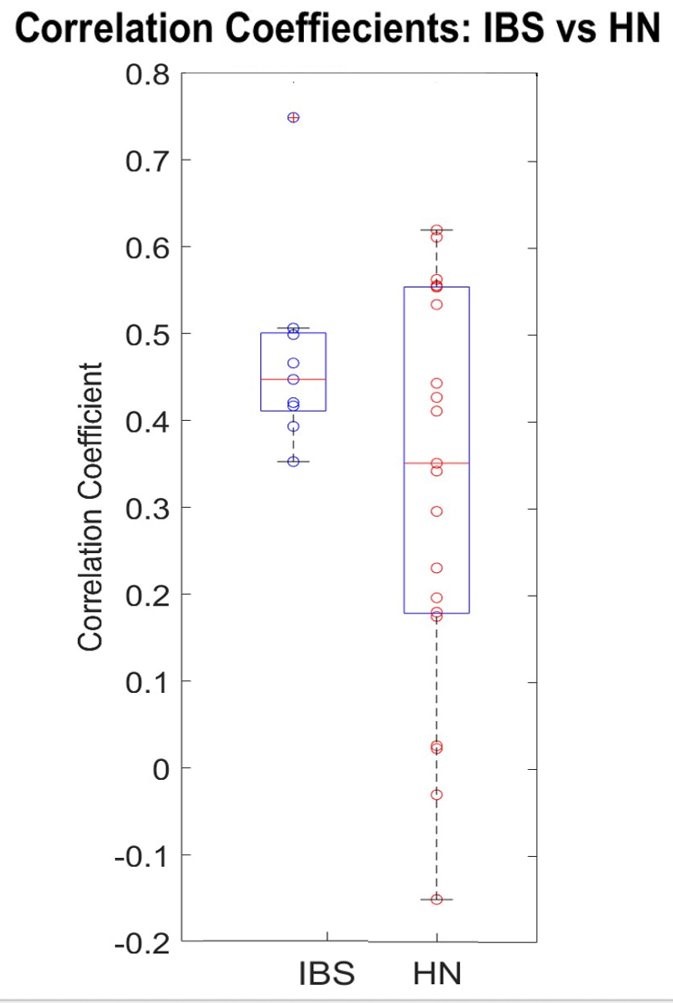
Analysis of the difference of colonic signal behavior between the two groups is ongoing, but early findings may indicate significant differences in temporal characteristics of the colonic responses and their correlation to heart rate variability. For example, Figure 5, which has been generated from preliminary data analysis in research, seems to suggest that correlations between heart rate (HR) and colonic activity are tighter for those with IBS. The healthy normals’ correlation coefficients display a greater spread, thus suggesting HR and colonic activity are not as linked. Though we have yet to definitively determine the reason behind the tight correlation in IBS subjects, it is hypothesized that there may be higher levels of nervous system input from subjects (Shaikh, 2023).
Signal strength and colonic activity generally tend to increase in the postprandial window, as demonstrated in Figures 2 and 4 (Erickson et al, 2019). The overall goal of this research is to eventually provide enough useful information to physicians to aid in noninvasive diagnosis of IBS, as well as to supply physicians with inexpensive monitoring tools that can be used to monitor for post-operative ileus. Additionally, we want to phenotype subgroups based on what may be driving the symptoms; some may have abnormal motility and others may have an overactive nervous response or a BGA disorder. These have different treatment plans and more information would assist doctors in deciding which to utilize when treating patients.


Current Multichannel Bioamplifier Module Design
Electronics
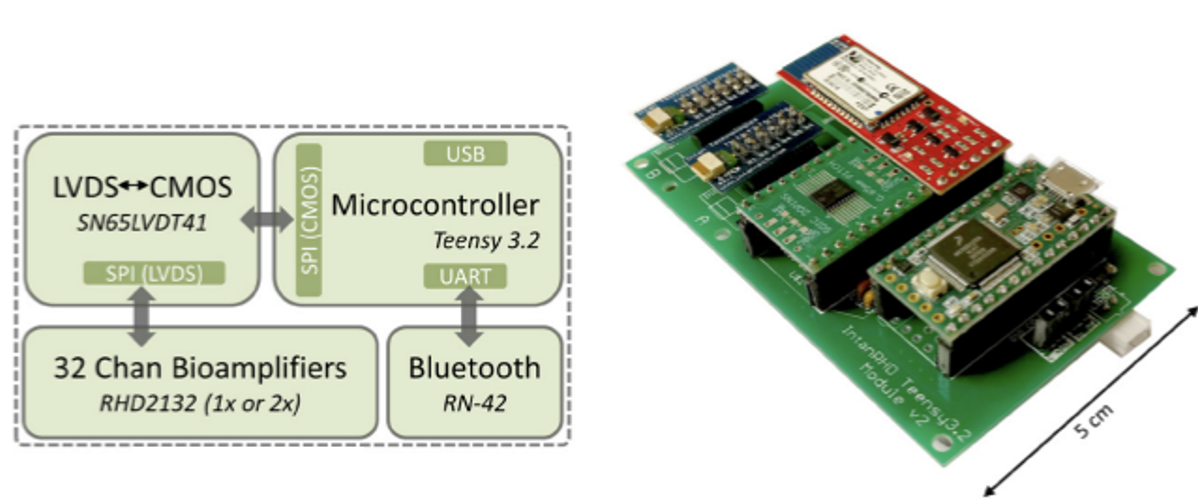
The multichannel bioamplifier module used in the experiments described above employs four main electrical components as illustrated in Figure 6 below. It utilizes the Teensy 3.6 MCU along with four 32-channel Intan RHD-2132 amplifiers. The four amplifiers and the microcontroller electronically interface via a SN65LVTD41 low-voltage differential signaling (LVDS) to complementary metal-oxide semiconductor (CMOS) transceiver. This is an important step as the MCU’s native language is CMOS and the bioamplifier has a native language of LVDS. The system also includes an RN-42 Bluetooth module for wireless real-time data streaming.
Housing Unit
The current device’s housing unit does not allow the participant to be comfortably and conveniently ambulatory. With its current size of 13.1 x 8.8 x 2.5 cm, the participant must remain in close proximity to the device, in a chair or carrying the device in a purse, for the time period of the testing. The box was 3D printed out of ABS plastic, which makes the device strong and allowed for the box shape to be customized to the electronic components. The box also has various access ports for easy access to the SD card, and the USB port to connect to the microcontroller.
Because of the profile of the housing unit, the device is not conveniently portable, which limits studies to 3-4 hours. Our goal is to redesign the current bioamplifier device to maximize comfort and permit portability. To minimize size, we are considering smaller electrical components and utilizing 32 electrodes rather than 64. The number of electrode channels relates to the number of electrodes the participant wears for the study. Previous versions of this device have included up to 128 channels, such as one described in a 2020 paper (Erickson et al, 2020). Previous studies have been successfully conducted with both 32 and 64 channels. Both amounts were able to effectively collect sufficient data to spatiotemporally map colon motility. (Erickson et al, 2018). For this design, we selected 32 channels because we believe it appropriately balances the ability to measure the colonic signal from the surface of the lower abdomen and patient comfort. Other options are 64 and 8 channels but we deemed this suboptimal. A device with 32 channels will provide high-enough resolution across the abdominal region to enable spatial mapping of colonic motility, an area of interest throughout IBS research, that would that would be substantially less feasible with a device containing only 8 electrodes (Gharibans et al, 2017)(Erickson et al, 2023).
